Answer:
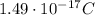
Step-by-step explanation:
The oil drop remains stationary when the electric force on it and the gravitational force are balanced, so we have:
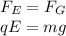
where
q is the charge of the oil drop
E is the electric field strength
m is the mass of the drop
g is the acceleration due to gravity
here we have
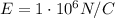
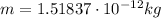
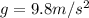
So the charge of the drop is
