Answer:
Heating this gas to 55 °C will raise its volume to 6.87 liters.
Assumption: this gas is ideal.
Step-by-step explanation:
By Charles's Law, under constant pressure the volume
 of an ideal gas is proportional to its absolute temperature
of an ideal gas is proportional to its absolute temperature
 (the one in degrees Kelvins.)
(the one in degrees Kelvins.)
Alternatively, consider the ideal gas law:
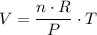 .
.
 is the number of moles of particles in this gas.
is the number of moles of particles in this gas.
 should be constant as long as the container does not leak.
should be constant as long as the container does not leak.
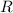 is the ideal gas constant.
is the ideal gas constant.
 is the pressure on the gas. The question states that the pressure on this gas is constant.
is the pressure on the gas. The question states that the pressure on this gas is constant.
Therefore the volume of the gas is proportional to its absolute temperature.
Either way,
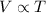 .
.
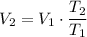 .
.
For the gas in this question:
- Initial volume:
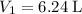 .
.
Convert the two temperatures to degrees Kelvins:
- Initial temperature:
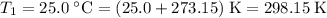 .
. - Final temperature:
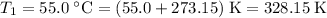 .
.
Apply Charles's Law:
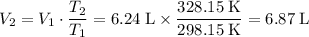 .
.