Answer:
155 kJ of energy will be released.
Step-by-step explanation:
The
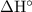 (
(
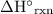 in some textbooks) here stands for standard enthalpy change per mole reaction. To find the amount of energy released in this reaction, start by finding the number of moles of this reaction that will take place.
in some textbooks) here stands for standard enthalpy change per mole reaction. To find the amount of energy released in this reaction, start by finding the number of moles of this reaction that will take place.
How many moles of atoms in 4.72 grams of carbon?
Relative atomic mass data from a modern periodic table:
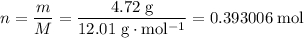 .
.
The coefficient of carbon in the equation is one. In other words, each mole of the reaction will consume one mole of carbon. Oxygen is in excess. As a result,
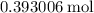 of carbon will support
of carbon will support
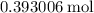 of the reaction.
of the reaction.
How much energy will be released?
The
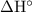 value here is negative. But don't panic.
value here is negative. But don't panic.
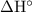 is the same as the chemical potential energy of the reactants minus the products in one mole of the reaction.
is the same as the chemical potential energy of the reactants minus the products in one mole of the reaction.
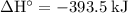 means that the chemical potential energy drops by
means that the chemical potential energy drops by
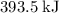 during each mole of the reaction (with the coefficients as-is.) Those energy difference will be released as heat. In other words, one mole of the reaction will release
during each mole of the reaction (with the coefficients as-is.) Those energy difference will be released as heat. In other words, one mole of the reaction will release
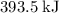 of energy.
of energy.
The 4.72 grams of carbon will support
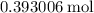 of this reaction. How much heat will that
of this reaction. How much heat will that
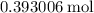 of reaction release?
of reaction release?
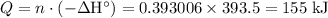 .
.
As a side note, the mass of carbon 4.72 grams is the least significant data in this question. There are three significant figures in this value. As a result, keep more than three significant figures in calculations but round the final result to three significant figures.