Answer:
4.5L
Step-by-step explanation:
Given parameters:
Inital volume, V₁ of the dry air = 1.50L
Initial pressure P₁ = 3.0atm
Temperature = 25°C
Final pressure P₂ = 1.0atm
Final volume of gas, V₂ = ?
To solve gas related problem, we apply Boyle's law based on the parameters given.
Boyle's law states that the volume of a fixed mass of gas is inversely proportional to the pressure provided that temperature is constant.
The law is simply expressed as:
P₁V₁ = P₂V₂
From the parameters given, the unknown V₂ which is the final volume.
Making V₂ the subject of the equation gives:
V₂ =
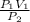
V₂ =
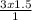 = 4.5L
= 4.5L