Answer:
12mLd
Step-by-step explanation:
Given parameters:
Concentration of base, NaOH = 2.0M
Volume of acid, HCl = 24mL = 24 x 10⁻³L = 0.024L
Concentration of HCl = 1.0M
Unknown parameter
Volume of NaOH = ?
The equation of the reaction is NaOH + HCl → NaCl + H₂O
Method
1. Starting the known values, we find the number of moles of acid used. From the reaction equation , we know that:
1 mole of NaOH reacts with 1 mole of HCl
We use the above to find the number of moles of base used
2. From the number of moles of base, we plug it into the equation below:
Volume of NaOH =
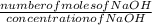
Solution
Number of moles of acid = concentration of acid x volume of acid
Number of moles = 1M x 0.024L = 0.024mol
From the balanced equation we know that:
1 mole of NaOH reacts with 1 mole of HCl
Therefore, the number of moles of NaOH is 0.024mol
Using the equation below, we have:
Volume of NaOH =
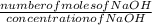
Volume of NaoH =
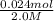
Volume of NaOH = 0.012L = 12mL