Answer: Arrow E represents the enthalpy of the reaction.
Step-by-step explanation:
Enthalpy of the reaction is defined as the difference in the potential energy of the products and the reactants. It is represented as
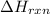
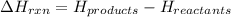
From the image, the points marked represents:
Point A represents the potential energy of the reactants.
Point B represents the intermediate state or transition state in a reaction.
Point C represents the potential energy of the products.
Arrow D represents the activation energy of the reaction.
Arrow E represents the enthalpy of the reaction.
Hence, arrow E represents the enthalpy of the reaction.