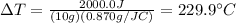Answer:
Step-by-step explanation:
The increase in temperature of a substance is given by
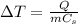
where
Q is the amount of heat absorbed by the substance
m is the mass of the substance
Cs is the specific heat capacity
In this problem,
Q = 2000.0 J
m = 10 g
Cs = 0.870 J/gC
Substituting, we find the increase in temperature: