Answer:
The volume would be 800mL and it shows that it is constant.
Step-by-step explanation:
Given parameters:
Intial volume of gas V₁ = 800mL
Initial temperature T₁ = -23°C to Kelvin; we use K = °C + 273 = -23 + 273 = 250K
Inital pressure P₁ = 300torr
Final temperature T₂ = 227°C to K = 227 + 273 = 500K
Final pressure P₂ = 600torr
Unknown parameter:
Final volume V₂ = ?
To solve this problem, we use the General gas law or the combined gas law where we assume that n=1, this gives:
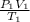 =
=
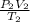
Since the unknown is V₂, we make it the subject of the formula:
V₂ =
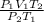
V₂ =
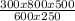
V₂ = 800mL