Answer:
15.9gmol⁻¹
The gas could have been methane when we compare the two molecular masses.
Oxygen gas, O₂ also shares this same number for its molecular mass. This would be the reason why it would not be methane.
Step-by-step explanation:
Given parameters:
Volume of gas at STP = 2.0L or 2.0dm³(1L = dm³)
Mass of the gas = 1.43g
Unknown:
Molecular mass of the gas = ?
Solution
We can find the number of moles using the mole concept according to the equation below:
number of moles = ³⁻¹
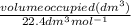
number of moles =
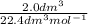
number of moles = 0.089mol
Using this obtained number of moles, we can derive the molecular mass of the compound by using the relationship between mass and number of moles according to the equation below:
molecular mass =
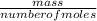
molecular mass =
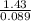
molecular mass of the gas = 15.9gmol⁻¹
The formula of methane gas is CH₄.
Let's calculate the molecular mass of methane using these atomic masses:
C = 12g and H = 1g
The molecular mass = 12 + (1x4) = 16gmol⁻¹
The gas could have been methane when we compare the two molecular masses.
Oxygen gas, O₂ also shares this same number for its molecular mass. This would be the reason why it would not be methane.