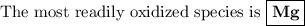Answer:
Step-by-step explanation:
Think of it this way.
The Mg half-reaction has the most negative potential, so it has the least tendency to go to the right.
For the same reason, it has the greatest tendency to go to the left.
The oxidation half-reaction would be
Mg ⟶ Mg²⁺ + 2e⁻ E° = 2.37 V