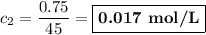Answer:
Step-by-step explanation:
Na₂S₂O₃ solution does not react with KI (it reacts with I₂), so it is simply diluting the KI, and we can use the dilution formula.
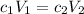
Data:
c₁ = 0.15 mol·L⁻¹; V₁ = 5 drops
V(Na₂S₂O₃) = 40 drops
Calculations:
(a) Calculate the total volume
V₂ = 5 + 40 = 45 drops
(b) Calculate the concentration
0.15 × 5 = c₂ × 45
0.75 = 45c₂