Answer:
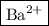
Step-by-step explanation:
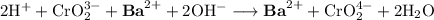
The spectator ions are the ions that are on both sides of the equation.
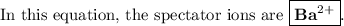
They are present at the beginning and at the end of the reaction. They don't take part in the reaction. They are simply "spectators" watching the other ions "do their thing."