Answer:
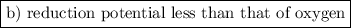
Step-by-step explanation:
Electrochemical corrosion of a metal is its spontaneous reaction with oxygen.
Let's compare the standard reduction potentials for a hypothetical metal M with that of oxygen.
E°/V
O₂(g) + 2H₂O(ℓ) + 4e⁻ ⇌ 4OH⁻(aq) 0.4
M²⁺(aq) + 2e⁻ ⇌ M(s) -0.1
To react spontaneously with O₂, the metal must have a

E°/V
2 × [M(s) ⇌ M²⁺(aq) + 2e⁻ ] 0.1
1 × [O₂(g) +2H₂O(ℓ) + 4e⁻ ⇌ 4OH⁻(aq)] 0.4
2M(s) + O₂(g) + 2H₂O(ℓ) ⇌ 2M²⁺(aq) + 4OH⁻(aq) 0.5
Only then will we get a positive for the overall cell potential and a spontaneous reaction.