Answer:

Step-by-step explanation:
To convert from moles to atoms, we must use Avogadro's Number.
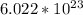
This number tells us the number of particles (atoms, molecules, ions, etc.) in 1 mole. In this case, the particles are atoms of calcium (Ca).
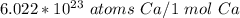
1. Convert from moles to atoms.
Write Avogadro's number as a fraction.
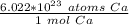
Multiply the given number of moles of calcium (3.8) by the fraction created.
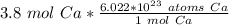
The moles of calcium will cancel.
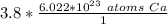
The denominator of 1 is insignifcant and we can turn this into a simple multiplication problem.
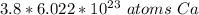
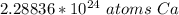
2. Round
The problem tells us to round to 2 decimal places or the hundredth place.
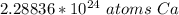
The 8 in the thousandth place tells us to round the 8 to a 9.
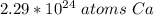
There are about 2.29*10²⁴ atoms of calcium in 3.8 moles.