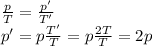Answer:
Gay Lussac law
Step-by-step explanation:
Gay Lussac law states that for a gas kept at constant volume (so, in a rigid container), the pressure of the gas is directly proportional to the absolute temperature.
In mathematical formula:
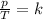
where
p is the gas pressure
T is the absolute temperature
According to this law, we see therefore that if the absolute temperature of the gas is doubled:
T' = 2T
The pressure will also double: