Answer: its temperature must increase.
Step-by-step explanation:
In an isobaric process the pressure remains constant, which means the initial pressure and the final pressure will be the same.
In addition, during this thermodynamic process, the volume of the ideal gas expands or contracts in such a way that the variation of pressure
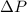 is neutralized.
is neutralized.
Now, according to the First law of Thermodynamics that establishes the conservation of energy:
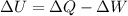 (1)
(1)
Where:
 is the internal energy
is the internal energy
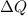 is the heat transferred
is the heat transferred
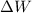 is the work
is the work
Now, for an isobaric process:
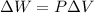 (2)
(2)
Where:
 is the pressure (always positive)
is the pressure (always positive)
 is the volume variation of the gas
is the volume variation of the gas
Here we have two possible results:
-If the gas expands (positive
 ), the work is positive.
), the work is positive.
-If the gas compresses (negative
 ), the work is negative.
), the work is negative.
In this case we are talking about the first result (work is positive).
Then, according to the above, equation (1) can be written as follows:
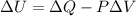 (3)
(3)
Clearing
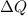 :
:
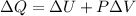 (4)
(4)
Then, for an ideal gas in an isobaric process, part of the heat (
 ) added to the system will be used to do work (positive in this case) and the other part will increase the internal energy, hence the temperature will increase as well.
) added to the system will be used to do work (positive in this case) and the other part will increase the internal energy, hence the temperature will increase as well.