Answer:
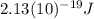
Step-by-step explanation:
The photoelectric effect consists of the emission of electrons (electric current) that occurs when light falls on a metal surface under certain conditions.
If the light is a stream of photons and each of them has energy, this energy is able to pull an electron out of the crystalline lattice of the metal and communicate, in addition, a kinetic energy.
This is what Einstein proposed:
Light behaves like a stream of particles called photons with an energy
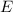
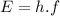 (1)
(1)
Where:
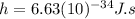 is the Planck constant
is the Planck constant
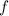 is the frequency
is the frequency
Now, the frequency has an inverse relation with the wavelength
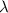 :
:
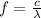 (2)
(2)
Where
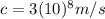 is the speed of light in vacuum and
is the speed of light in vacuum and
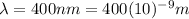 is the wavelength of the absorbed photons in the photoelectric effect.
is the wavelength of the absorbed photons in the photoelectric effect.
Substituting (2) in (1):
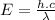 (3)
(3)
So, the energy
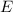 of the incident photon must be equal to the sum of the Work function
of the incident photon must be equal to the sum of the Work function
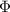 of the metal and the maximum kinetic energy
of the metal and the maximum kinetic energy
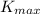 of the photoelectron:
of the photoelectron:
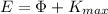 (4)
(4)
Rewriting to find
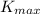 :
:
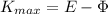 (5)
(5)
Where
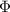 is the minimum amount of energy required to induce the photoemission of electrons from the surface of a metal, and its value depends on the metal:
is the minimum amount of energy required to induce the photoemission of electrons from the surface of a metal, and its value depends on the metal:
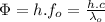 (6)
(6)
Being
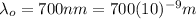 the threshold wavelength (the minimum wavelength needed to initiate the photoelectric effect)
the threshold wavelength (the minimum wavelength needed to initiate the photoelectric effect)
Substituting (3) and (6) in (5):
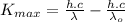
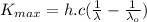 (7)
(7)
Substituting the known values:
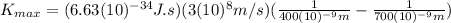
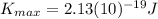 >>>>>This is the maximum kinetic energy that ejected electrons must have when violet light illuminates the material
>>>>>This is the maximum kinetic energy that ejected electrons must have when violet light illuminates the material