Answer:
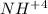 is a A. Polyatomic ion
is a A. Polyatomic ion
Step-by-step explanation:
When two atoms combine with each other through a covalent bond it leads to the formation of charged species called as polyatomic ions.
The ammonium cation is a decidedly accused polyatomic particle of the synthetic recipe
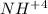 . It is shaped by the protonation of ammonia. Ammonium is additionally a general name for decidedly charged or protonated substituted amines and quaternary ammonium cations
. It is shaped by the protonation of ammonia. Ammonium is additionally a general name for decidedly charged or protonated substituted amines and quaternary ammonium cations
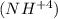 , where at least one hydrogen molecules are supplanted by natural gatherings.
, where at least one hydrogen molecules are supplanted by natural gatherings.