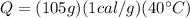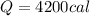Answer: 4200 calories
Let's start by explaining that 1 Calorie (
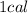 ) is defined as a unit of thermal energy that is equivalent to the amount of heat (
) is defined as a unit of thermal energy that is equivalent to the amount of heat (
 ) needed to raise the temperature of 1 gram
) needed to raise the temperature of 1 gram
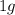 of water by 1 degree Celsius
of water by 1 degree Celsius
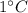 .
.
The formula to calculate it is:
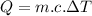 (1)
(1)
Where:
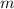 is the mass
is the mass
 is the specific heat of the element. In the case of water
is the specific heat of the element. In the case of water
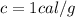
 is the variation in temperature, which in this case is
is the variation in temperature, which in this case is
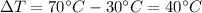
Rewriting equation (1) with the known values: