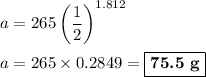Answer:
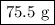
Explanation:
The amount of substance decreases by 50 % every half-life.
Thus, 50 % of the substance remains at the end of each half-life.
The exponential function is
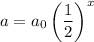
where x = the number of half-lives.
Data:
a₀ = 265 g
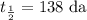
t = 250 da
Calculations:
(a) Calculate x
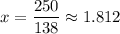
(b) Calculate a