Answer: Option (a) is the correct answer.
Step-by-step explanation:
A bond where transfer of electrons take place from one atom to another is known as an ionic bond.
An ionic solution is defined as a solution in which an ionic compound is dissociated into ions.
For example, NaCl is an ionic compound and when it is dissolved in water it dissociates as
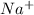 and
and
 ions.
ions.
As it is known that electric current is the flow of charges. Hence, a solution that contains ions will be a good conductor of electricity.
Whereas in general, a covalent solution does not contain any ions.
Thus, we can conclude that ionic solutions are good conductors of electricity.