Answer : The correct option is,
Explanation :
The formula used for the percent yield will be :
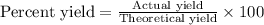
or,
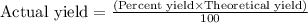
For example : If we are given that the percentage yield of a sample is 94.92% and the theoretical yield is 83.475 g. Now calculate the actual yield of the sample.
By using formula we get the value of actual yield.
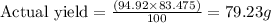
Thus, the actual yield is, 79.23 g.
Hence, the formula used to calculate the actual yield can be,