Answer:
70mol
Step-by-step explanation:
The equation of the reaction is given as:
2C₂H₂ + 5O₂ → 4CO₂ + 2H₂O
Given parameters:
Number of moles of acetylene = 35.0mol
Number of moles of oxygen in the tank = 84.0mol
Unknown:
Number of moles of CO₂ produced = 35.0mol
Solution:
From the information given about the reaction, we know that the reactant that limits this combustion process is acetylene. Oxygen is given in excess and we don't know the number of moles of this gas that was used up. We know for sure that all the moles of acetylene provided was used to furnish the burning procedure.
To determine the number of moles of CO₂ produced, we use the stoichiometric relationship between the known acetylene and the CO₂ produced from the balanced chemical equation:
From the equation:
2 moles of acetylene produced 4 moles of CO₂
∴ 35.0 mol of acetylene would produced:
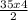 = 70mol
= 70mol