Answer: 450 Bq
Step-by-step explanation:
This problem can be solved using the Radioactive Half Life Formula:
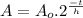 (1)
(1)
Where:
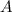 is the final amount of radioisotope (decay rate)
is the final amount of radioisotope (decay rate)
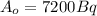 is the initial amount of the radioisotope
is the initial amount of the radioisotope
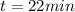 is the time elapsed
is the time elapsed
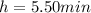 is the half life of the radioisotope
is the half life of the radioisotope
Knowing this, let's find
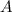 from (1):
from (1):
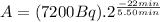 (2)
(2)
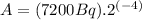
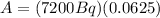 (3)
(3)
Finally:
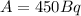 >>> This is the decay rate of the radioisotope
>>> This is the decay rate of the radioisotope
Note it is in Becquerels (Bq), which is the derived unit approved by the International System of Units for radioactivity