Hello!
The answer is:
The first measurement of volume is equal to 835.23 mL.
Why?
Boyle's Law equation can be used when the temperature is kept constant, and it establishes a relation between the pressure and volume, showing that when an ideal gas is kept constant, the pressure and volume are inversely proportional.
So, we Boyle's Law equation states that:
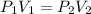
Where,
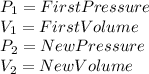
Now, if we are looking for the first volume measurement, we need to rewrite the equation as follow:
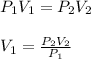
So, substituting the given information and calculating, we have:
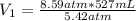
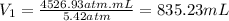
Hence, the first measurement of volume is equal 835.23 mL.
Have a nice day!