Answer:
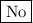
Step-by-step explanation:
Na reacts with all OH groups to produce hydrogen.
The reaction with cyclobutanol is shown below.
The reaction with butanoic acid is
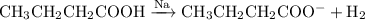
Both compounds release bubbles of hydrogen, so you can't use sodium to distinguish between them.