Answer:
Carbonic acid
Step-by-step explanation:
Hello,
In this case, due to the increasing concentration of carbon dioxide in the atmosphere, when it rains, the following chemical reaction occurs between water and carbon dioxide:
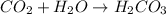
Whose product is known as carbonic acid and it is the natural source of acidity in acid rain.
Regards.