A) Francium-223
In an alpha decay, a nucleus decay emitting an alpha particle, which corresponds to a nucleus of helium: so, it consists of 2 protons and 2 neutrons.
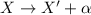
This means that in the decay:
- The original nucleus loses 2 protons --> so its atomic number Z decreases by 2 units
- The original nucleus loses 2 nucleons (2 protons and 2 neutrons) --> so its mass number A decreases by 4 units
In this example, the original nucleus is Ac (Actinium), with
Z = 89
A = 227
After the decay, it must be
Z - 2 = 89 - 2 = 87
A - 4 = 227 - 4 = 223
We see from the periodict table, Z=87 corresponds to Francium (Fr), so the final nucleus will be francium-223 (the isotope of francium with 223 nucleons).
B) Polonium-211
In a beta-minus decay, a neutron in the nucleus turns into a proton, emitting a fast-moving electron (the beta particle) and an anti-neutrino.
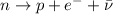
Therefore, in this process:
- The original nucleus gains 1 protons, so its atomic number Z increases by 1 unit
- The original nucleus does not lose/gain nucleons, so its mass number A remains the same
In this example, the original nucleus is Bi (bismuth)-211, with
Z = 83
A = 211
So After the decay, it will be
Z + 1 = 83 + 1 = 84
A = 211
So, the nucleus will be Polonium (Z=84), isotope with 211 nucleons.
C) Neon-22
In a beta-plus decay, a proton in the nucleus turns into a neutron, emitting a fast-moving positron (the beta particle) and a neutrino.
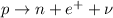
Therefore, in this process:
- The original nucleus loses 1 protons, so its atomic number Z decreases by 1 unit
- The original nucleus does not lose/gain nucleons, so its mass number A remains the same
In this example, the original nucleus is Na (sodium)-22, with
Z = 11
A = 22
So After the decay, it will be
Z - 1 = 11 - 1 = 10
A = 22
So, the nucleus will be Neon (Z=10), isotope with 22 nucleons.
D) Technetium-98
In a gamma decay, an unstable nucleus emits a gamma ray:
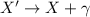
In this process, only energy is released (in the form of gamma ray), so there is no gain/loss of protons/neutrons in the process. This means that:
- The atomic number Z remains constant
- The mass number A remains constant
In this example, we have a nucleus of Tc (Technetium)-98, with
Z = 43
A = 98
These numbers will not change during the decay: this means that after the decay, we will still have a nucleus of Technetium-98.