Answer:
14.68 moles of He
Step-by-step explanation:
To do this, just remember Avogadro's Constant or Avogadro's number. This constant tells us how many units ( in this case atoms) there are in a mole of ANY type of substance.
Avogadro's constant is 6.022140857 × 10²³ units per mole.
Now that we know how many atoms there are in 1 mole, we can use this as our conversion factor.
8.84 x 10²⁴ atoms of He → moles of He
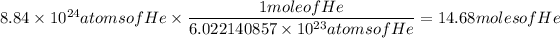
So the answer would be:
14.68 moles of He