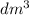Answer:
228.7
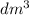
Step-by-step explanation:
According to Boyle's law
P1V1 = P2V2
where P1& P2 are initial and final pressure
and V1& V2 and initial and final volumes respectively.
Given:
P1 = 136atm, P2 = 131.6atm
V1 = 142
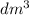 ; V2 = ?
; V2 = ?
V2 =
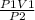 =
=
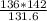
= 146.7
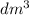
Total volume, V = V1 + V2
=146.7 + 142
= 228.7