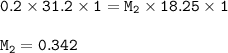The molar concentration of the original HF solution : 0.342 M
Further explanation
Given
31.2 ml of 0.200 M NaOH
18.2 ml of HF
Required
The molar concentration of HF
Solution
Titration formula
M₁V₁n₁=M₂V₂n₂
n=acid/base valence (amount of H⁺/OH⁻, for NaOH and HF n =1)
Input the value :