pH of solution = 13.033
Further explanation
Given
2.31 g Ba(OH)₂
250 ml water
Required
pH of solution
Solution
Barium hydroxide is fully ionized, means that Ba(OH)₂ is a strong base
So we use a strong base formula to find the pH
[OH ⁻] = b. Mb where
b = number of OH⁻ /base valence
Mb = strong base concentration
Molarity of Ba(OH)₂(MW=171.34 g/mol) :
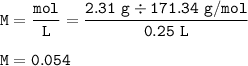
Ba(OH)₂ ⇒ Ba²⁺ + 2OH⁻(b=valence=2)
[OH⁻]= 2 . 0.054
[OH⁻] = 0.108
pOH= - log 0.108
pOH=0.967
pOH+pH=14
pH=14-0.967
pH=13.033