1. 13,500 cal
First of all, we need to find the amount of heat needed to raise the temperature of the ice from -20°C to 0°C. This is given by
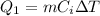
where
m = 150 g is the mass of the ice
C_i = 0.5 cal/g·C° is the specific heat capacity of the ice
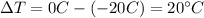 is the change in temperature of the ice
is the change in temperature of the ice
Substituting,
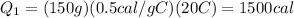
Now we have to find the amount of heat needed to melt the ice, which is
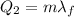
where
m = 150 g is the mass of the ice
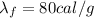 is the latent heat of fusion
is the latent heat of fusion
Substituting,
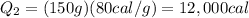
So the total heat required is
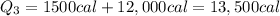
2. 3750 cal
The additional amount of heat required to heat the water to 25°C is
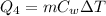
where
m = 150 g is the mass of water
C_w = 1 cal/g·C is the speficic heat capacity of water
 is the change in temperature
is the change in temperature
Substituting,
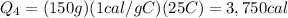
3. 9200 cal
First of all, we need to find the amount of heat needed to raise the temperature of the ice from -20°C to 0°C. As at point 1., this is given by
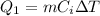
where
m = 80 g is the mass of the ice
C_i = 0.5 cal/g·C° is the specific heat capacity of the ice
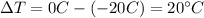 is the change in temperature of the ice
is the change in temperature of the ice
Substituting,
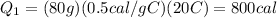
Now we have to find the amount of heat needed to melt the ice:
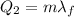
where
m = 80 g is the mass of the ice
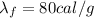 is the latent heat of fusion
is the latent heat of fusion
Substituting,
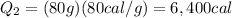
Finally, the amount of heat required to heat the water to 25°C is
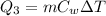
where
m = 80 g is the mass of water
C_w = 1 cal/g·C is the speficic heat capacity of water
 is the change in temperature
is the change in temperature
Substituting,
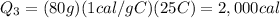
So the total heat required is
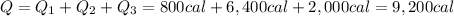
4. No
Step-by-step explanation:
The total heat required for this process consists of 3 different amounts of heat:
1- The heat required to bring the ice at melting temperature
2- The heat required to melt the ice, while its temperature stays constant
3- The heat required to raise the temperature of the water
However, computing how much heat is required to melt the ice and adding the amount of heat required to raise the temperature of 80 g of water by 45°C is not equivalent: in fact, the calculation of point 1) requires to use the specific heat capacity of ice, not that of water, therefore the two are not equivalent.