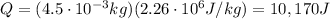1. 408.4 J
The work done by a gas is given by:
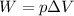
where
p is the gas pressure
 is the change in volume of the gas
is the change in volume of the gas
In this problem,
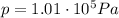 (atmospheric pressure)
(atmospheric pressure)
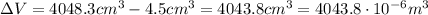 is the change in volume
is the change in volume
So, the work done is
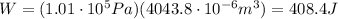
2. 10170 J
The amount of heat added to the water to completely boil it is equal to the latent heat of vaporization:
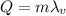
where
m is the mass of the water
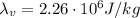 is the specific latent heat of vaporization
is the specific latent heat of vaporization
The initial volume of water is
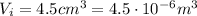
and the water density is
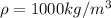
So the water mass is
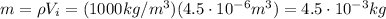
So, the amount of heat added to the water is