Answer:
The lithium atom has one electron in its outer shell, so it loses that one electron and becomes a
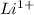 ion. The fluorine atom has 7 electrons in its outer shell so it gains that one electron and becomes a
ion. The fluorine atom has 7 electrons in its outer shell so it gains that one electron and becomes a
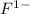 ion. The electrostatic force of attraction between the two ions, because of their charges, bonds them together in an ionic bond to make
ion. The electrostatic force of attraction between the two ions, because of their charges, bonds them together in an ionic bond to make
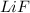 , Lithium Fluoride.
, Lithium Fluoride.
Couldn't add a diagram, but hope it helps.