Answer:
10.1 % Carbon, 0.8 % Hydrogen and 89.1 % Chlorine.
Step-by-step explanation:
The total mass of the compound is 5.34 + 0.42 + 47.08 = 52.84 g.
The percentage of carbon is:
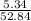 × 100 = 10.1 %
× 100 = 10.1 %
The percentage of hydrogen is:
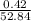 × 100 = 0.8 %
× 100 = 0.8 %
The percentage if chlorine is:
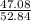 × 100 = 89.1 %
× 100 = 89.1 %