Answer:
.18 M
Step-by-step explanation:
1st
The molarity of an aqueous solution (a solution that has water as the solvent) can be found just by remember this one formula =
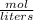 .
.
[moles of solute / volume of solution in L]
So, what do we have? From a glance we have the number of solute (Potassium Chromate), that tells us that we need to convert that value that is in grams (g) into...Moles or (mol), we can do that like this
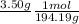
After we cross multiply 3.50 times 1 and then divide by 194.19 - we get this value ⇒ .018 mol (the g cancels, and we keep mol)
(We get the 194.19 from adding up the molecular weight of Potassium Chromate, which is about 194.19 g/mol. In the above equation though, we flipped this, so that 1 mol is in the numerator and the 194.19 is in the denominator; this helps us convert the 3.50 g to moles).
2nd
Now, we just have to remember that 1 liter = 1000 ml. Because its easier for us to use a L instead of "ml" in the molarity formula. So...
100.00 ml = .1 L ....
(because
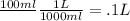
Finally, we just add our (.018 mol and .1 L) into one nice equation (plug the numbers we converted to back in)
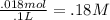
Is it clear now?