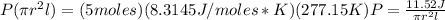Answer:
Step-by-step explanation:
You didn't ask a question here, but I assume you want the pressure? You can use the ideal gas equation stating that:
PV = nRT
The 'P' is pressure, the 'V' is volume, the 'n is number of moles of gas, the 'R' is the ideal gas constant, and the 'T' is the temperature. First convert the Celsius degrees into Kelvin by using the following relation:
C + 273.15 = K
4 + 273.15 = 277.15K
Now, find the volume of a cylinder by using:
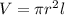
The 'l' is the length of the cylinder
Now just plug and chug everything into the ideal gas equation: