Answer: acid dissociation
Step-by-step explanation:
According to the Bronsted-Lowry conjugate acid-base theory, an acid is defined as a substance which looses donates protons and thus forming conjugate base and a base is defined as a substance which accepts protons and thus forming conjugate acid.
For the given chemical equation:
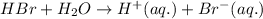
Here,
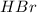 is loosing a proton, thus it is considered as an acid and the reaction is acid dissociation.
is loosing a proton, thus it is considered as an acid and the reaction is acid dissociation.
Hydroium ion is
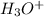
Neutralization reaction is a chemical reaction in which an acid reacts with base to produce salt and water.

Thus the reaction is acid dissociation.