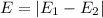Answer:
undergoes a transition to a quantum state of lower energy
Step-by-step explanation:
When electrons in an atom move to another quantum state, they emit/absorb a photon according to the following:
- If the electron is moving to a higher energy state, it absorbs a photon (because it needs energy to move to a higher energy level, so it must absorb the energy of the photon)
- if the electron is moving to a lower energy state, it emits a photon (because it releases the excess energy)
In particular, the energy of the absorbed/emitted photon is exactly equal to the difference in energy between the two levels of the electron transition: