Answer:
Molarity of the sulfuric acid is Choice C): 0.200 M.
Step-by-step explanation:
The unit for molarity "M" stands for moles per liter. Convert all volume to liters:
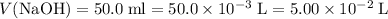 ;
;
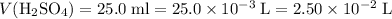 .
.
How many moles of potassium hydroxide is required to reach the equivalence endpoint?
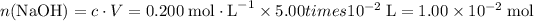 .
.
How many moles of sulfuric acid in the original solution?
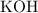 neutralizes
neutralizes
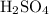 at a two-to-one ratio:
at a two-to-one ratio:
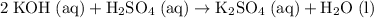 .
.
The
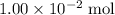 moles of
moles of
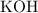 will neutralize only half as much
will neutralize only half as much
 . That is:
. That is:
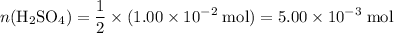 .
.
What's the molarity of the
 solution?
solution?
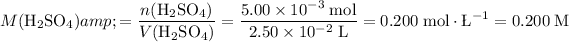 .
.