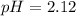Answer: pH = 2.12
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
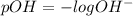
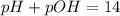
Give :
![[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/jptxicd74sqji6oqiawrnboqrtetqtax3v.png) = 0.00750
= 0.00750
![pH=-log[0.00750]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/af1ebupuragwnzhsdq4z3vxp2ue39a7x2e.png)