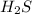Answer:
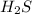 is a polar substance.
is a polar substance.
Step-by-step explanation:
Polar particles form when two molecules don't share electrons similarly in a covalent bond. This happens when there is a distinction between the electronegativity of every molecule. An extraordinary distinction shapes an ionic bond, while a lesser contrast frames a polar covalent bond.
If the electronegativity between the two ions is somewhere in the range of 0.5 and 2.0, the structure of the particle forms a polar covalent bond. In the event that the electronegativity distinction between the ions is more than 2.0, the bond is ionic. Ionic mixes are amazingly polar particles.
Instances of polar particles include:
- Water -

- Ammonia salts -

- Sulfur dioxide -
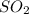
- Hydrogen sulfide -