Answer:
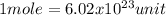 (Avogadro constant)
(Avogadro constant)
Step-by-step explanation:
It is known by the name of mole (mol) to one of the fundamental physical magnitudes that contemplate the International System of Units. This unit is used to measure the quantity of all kinds of substances present in a given system.
The mole, experts say, reflects the amount of substance that has a specific number of entities of elementary character as atoms can be found in twelve grams of carbon-12. This means that the number of elementary units (as in the case of atoms, molecules or ions, for example) that are reflected in a mole of substance is a constant that has no direct relationship with the type of particle or material in question. This amount is known as Avogadro's number.
This constant, baptized in homage to the scientist of Italian origin Amedeo Avogadro (1776-1856), allows to count microscopic particles from macroscopic measurements (as it is the case of the mass).