Answer:
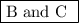
Step-by-step explanation:
In a single-displacement reaction, one element exchanges partners with another element in a compound.

This is a single-displacement reaction, because the element Fe exchanges partners with H in HCl.
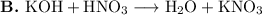
This is not a single-displacement reaction, because it is a reaction between two compounds.
This is a double displacement reaction in which the K⁺ and H⁺ cations change partners with the anions.
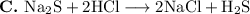
This is not a single-displacement reaction. It is another double displacement reaction, in which the Na⁺ and H⁺ cations change partners with the anions.

This is a single-displacement reaction, because the element Ca exchanges partners with H in H₂O.
 are not single-displacement reactions.
are not single-displacement reactions.