Answer:
the gas does no work.
Step-by-step explanation:
An isochoric process is a process in which the volume of a gas is kept constant.
The work done by a gas during a transformation is given by:
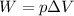
where
p is the gas pressure
 is the change in volume of the gas
is the change in volume of the gas
For an isochoric process, the volume of the gas does not change, so

and so, according to the previous equation, the work done by the gas is zero:
W = 0