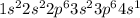Answer:
The answer is: Potassium because is the first element in period four.
Step-by-step explanation:
Ok, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. Since 1s can only hold two electrons, the next 2 electrons are in the 2s orbital. Then put six in the 2p orbital and put the next two electrons in the 3s. Now, move to the 3p where it is place the next six electrons. Then shift to the 4s orbital where we place the remaining one electron. Therefore the Potassium electron configuration will be: