Answer:
b) It is impossible to tell without knowing the masses.
Step-by-step explanation:
The temperature change of a substance when it receives/gives off a certain amount of heat Q is given by
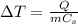
where
Q is the amount of heat
m is the mass of the substance
Cs is the specific heat capacity of the substance
In this case, we have a hot piece of aluminum in contact with a cold piece of copper: the amount of heat given off by the aluminum is equal to the amount of heat absorbed by the copper, so Q is the same for the two substances. However, we see that the temperature change of the two substances depends on two other factors: the mass, m, and the specific heat, Cs. So, since we know only the specific heat of the two substances, but not their mass, we can't tell which object will experience the greater temperature change.