Answer: The mass of gold is 2316 grams.
Step-by-step explanation:
To calculate the volume of cuboid, we use the equation:
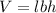
where,
V = volume of cuboid
l = length of cuboid = 10 cm
b = breadth of cuboid = 4 cm
h = height of cuboid = 3 cm
Putting values in above equation, we get:
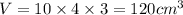
To calculate mass of a substance, we use the equation:

Putting values in above equation, we get:
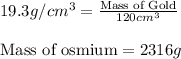
Hence, the mass of gold is 2316 grams.