Answer:
The solution with a pOH of 11 will be acidic in nature.
Step-by-step explanation:
When the aqueous solution is dissociated based upon the ions released by them it is categorized as acids or bases. When the solution gives out
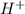 ion it is called as acid, whereas if it gives out
ion it is called as acid, whereas if it gives out
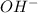 ions it is called as bases.
ions it is called as bases.
The concentration of
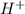 ions is represented by pH, whereas the concentration of
ions is represented by pH, whereas the concentration of
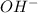 ion is represented by pOH. In pOH scale value seven refers to neutral, whereas value below seven shows that the substance is basic whereas the value above seven indicates the substance as acidic.
ion is represented by pOH. In pOH scale value seven refers to neutral, whereas value below seven shows that the substance is basic whereas the value above seven indicates the substance as acidic.
Thus, it can be inferred that the solution with a pOH of 11 will be acidic in nature.